The BF-Finnish Electrophysiology Platform services include:
- Open access to all facilities (after user training)
- Personal user support and general support activities: e.g., user training, consultation on experimental set up, sample preparation, choice of instruments, and data analysis
- Full-service model including experimental design, conduction of the measurements and analyses, preparation of results to e.g., scientific articles.
- Maintenance and development of equipment and methods
Jointly develop algorithms and software for advanced data analysis - Teaching activities: lectures and practical courses for MSc & graduate students & postdoctoral fellows
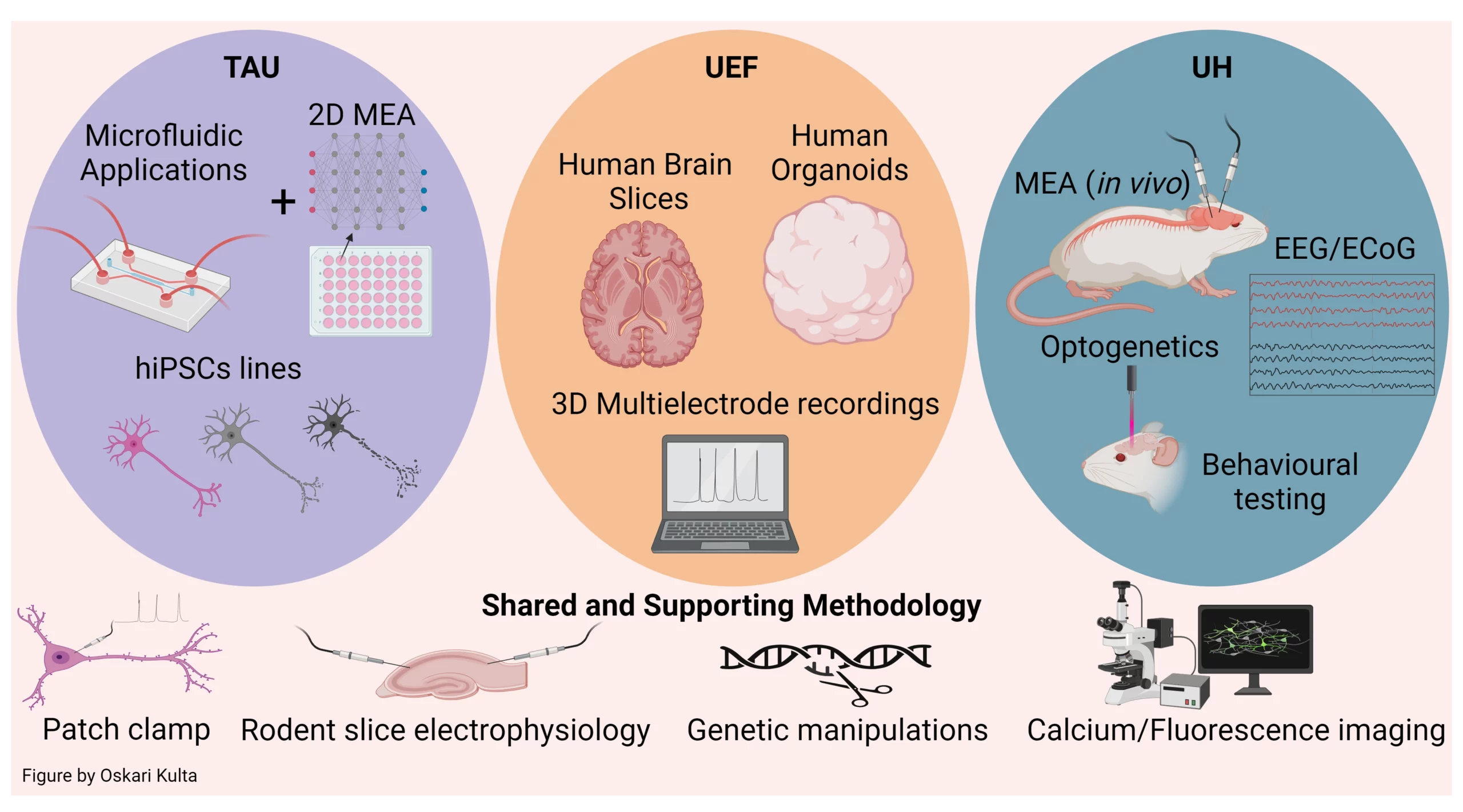
Image created with BioRender.com.
Nodes
TAU: Tampere University; UEF: University of Eastern Finland; UH: University of Helsinki
Contact details
Platform Chair
Susanna Narkilahti
susanna.narkilahti@tuni.fi
Tampere University
Services
FinE-UEF, Core facility of in vitro and ex vivo electrophysiology
The core facility carries out electrophysiological measurements of human stem cell derived models and brain biopsies obtained from surgeries. The core is specialized on 3D samples and cellular models but has capacity to record from 2D cultures. The core instrumentation includes 2 PC systems, one regular MEA system and one high density MEA system, and one fast fluorescence system.
FinE-UH, Helsinki Electrophysiology Platform
The core facility provides facilities and services in various electrophysiological techniques, including PC, field potential and MEA recordings from acutely isolated and cultured neuronal preparations, as well as from rodent brain in vivo. The instrumentation includes visually guided PC setups, MEA systems for neuronal cultures and for head-fixed awake mice. Recordings can be combined with behavioral tasks, pharmacological and genetic manipulation as well as electrical and optogenetic stimulation.

Photographs by Jonne Renvall
Recent user publications
Scoyni F, Giudice L, Väänänen MA, Downes N, Korhonen P, Choo XY, Välimäki NN, Mäkinen P, Korvenlaita N, Rozemuller AJ, de Vries HE, Polo J, Turunen TA, Ylä-Herttuala S, Hansen TB, Grubman A, Kaikkonen MU, Malm T. Alzheimer's disease-induced phagocytic microglia express a specific profile of coding and non-coding RNAs. Alzheimer’s & Dementia 2023 Oct 12. doi: 10.1002/alz.13502
Ignatova, I.; Frolov, R.; Nymark, S. The Retinal Pigment Epithelium Displays Electrical Excitability and Lateral Signal Spreading. BMC Biology 2023, 21 (1), 84. https://doi.org/10.1186/s12915-023-01559-5.
Gazestani V, Kamath T, Nadaf NM, Dougalis A, Burris SJ, Rooney B, Junkkari A, Vanderburg C, Pelkonen A, Gomez-Budia M, Välimäki NN, Rauramaa T, Therrien M, Koivisto AM, Tegtmeyer M, Herukka SK, Abdulraouf A, Marsh SE, Hiltunen M, Nehme R, Malm T, Stevens B, Leinonen V, Macosko EZ. Early Alzheimer’s disease pathology in human cortex involves transient cell states. Cell. 2023 Sep 28;186(20)4438-4453. e23. doi: 10.1016/j.cell.2023.08.005.
Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, Biojone C, Cannarozzo C, Sahu MP, Kaurinkoski K, Brunello CA, Steinzeig A, Winkel F, Patil S, Vestring S, Serchov T, Diniz CRAF. Laukkanen L, Cardon I, Antila H, Rog T, Piepponen TP, Bramham CR, Normann C, Lauri SE, Saarma M, Vattulainen I, Castren E. (2021) Antidepressants act by directly binding to the TRKB neurotrophin receptors. Cell, 184(5):1299-1313.e19


Photographs by Jonne Renvall


